What is the scope of IEC62304?
25/06/2020

With changes in new technologies and the need to digitise our environments, more and more medical devices (MDs) are integrating or taking the form of software programs. Regulation (EU) 2017/745 addresses this issue and sets a framework for these types of medical devices. When developing software, IEC 62304 is one of the standards to apply as it defines the life cycle requirements for the development of medical software and software within medical devices.
What is the scope of IEC 62304?
Standard IEC 62304 – Medical device software – Software life cycle processes applies to the development and maintenance of any software that is a medical device or is intended to be an integral part of a medical device.
Software is considered a medical device if it is intended for one or more of the medical purposes defined in REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL.
It is important to note that any software that does not have a medical purpose, such as a sports or nutrition management application, does not fall within the scope of the MD regulation.
This standard does not cover the validation and final release of the medical device, even when the device consists entirely of software. Depending on your MD, other standards may need to be applied such as IEC 82304 on health software (does not apply to health software intended to become an integral part of specific hardware designed for healthcare use) or IEC 60601-1 on medical electrical equipment.
Application oh l’IEC 62304 ?
The processes covered by the IEC 62304 standard are shown in the following diagram:
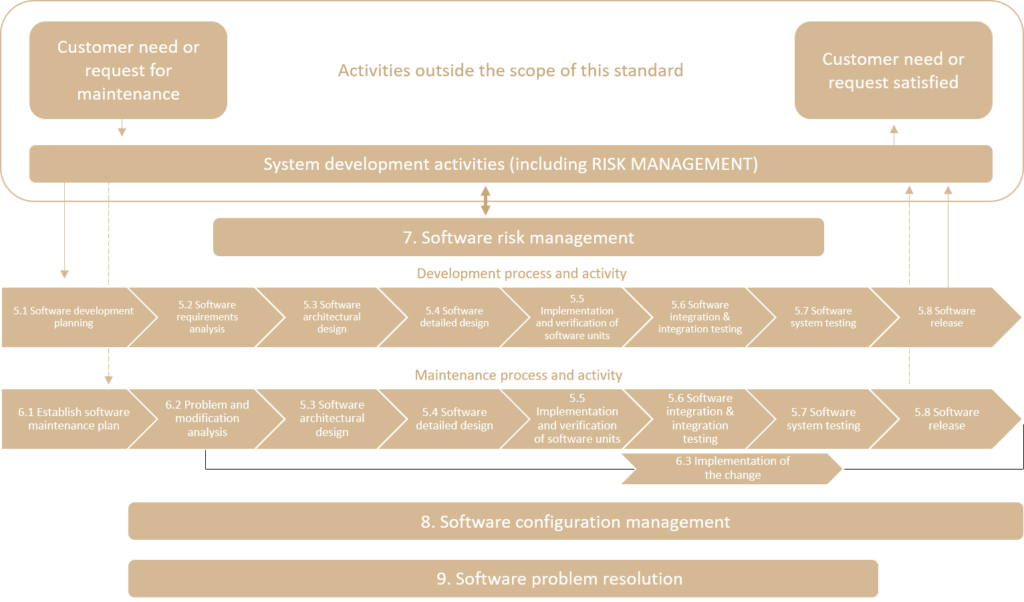
To apply the IEC 62304 standard, the following general requirements must be met:
- Implement a quality management system (ISO 13485)
- Implement a risk management process in accordance with ISO 14971
- Assign a safety class for software
IEC 62304 introduces three safety classes for software, ranging from A (least critical) to C (most critical); they are based on the severity of the consequences of a software failure:
- Class A: No injury or damage to health is possible
- Class B: Non-serious injury is possible
- Class C: Death or serious injury is possible
Note that depending on the class of the software, some (for Class A and B software) or all (for Class C software) of the requirements of IEC 62304 will have to be implemented (or monitored).
These software safety classes are independent of the four classes of medical devices as defined by Directive 93/42/EEC and Regulation (EU) 2017/745 (I, IIa, IIb and III).
Compliance with the standard requires development based on a robust risk analysis and a traceability system to ensure that the device is tracked throughout its life cycle.
Applying the IEC 62304 standard enables the applicable regulatory requirements to be met for the design, development, verification and maintenance of software.
If you would like more information concerning the content or application of this standard, please use the contact form.
Why should you turn to Efor for assistance?
Our regulatory affairs experts will be pleased to offer you high-quality support with regard to all of the following topics:
- Regulatory and standards monitoring to identify the regulations and standards applicable to your product.
- Regulatory compliance of your medical device (software or medical device integrating software) during the development phase.
- Assistance in drafting the product design file in accordance with the requirements of IEC 62304, ISO 14971, ISO 13485 and other applicable standards.
- Drafting of the technical dossier for the medical device.
Our digital experts will be pleased to provide you with support for the following technical verification activities:
- Planning and management of verification tests.
- Implementation and verification of software units.
- Integration of software units and testing of integrated software, including regression testing.
Efor group
Our CSR commitments
Aware of our social and environmental responsibility, we act every day to make a positive impact on society.
Nos actualités
Suivez toutes nos infos santé


