UDI : Keys dates
29/09/2021

One of the new features of Regulation (EU) 2017/745 (MDR) and Regulation (EU) 2017/746 (IVDR) is the implementation of the UDI system, in order to strengthen the traceability of medical devices (MDs) and in vitro diagnostic medical devices (IVDMDs).
When should you create these codes? When should you affix them to your MDs and IVDMDs? When should you enter them in Eudamed? What happens next?
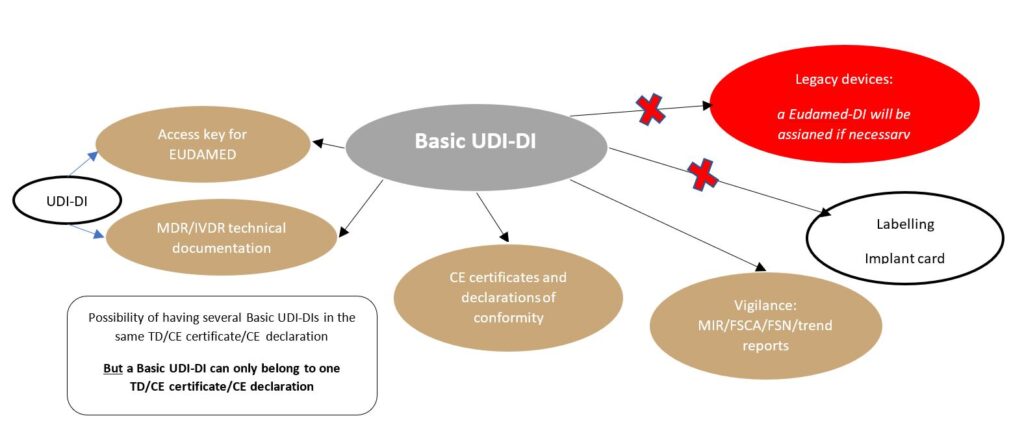
It is first necessary to distinguish between the Basic UDI-DI and the UDI.
L’UDI is an identifier meant to be affixed to devices
UDI = UDI+DI + UDI-PI
UDI-DI = “device” identification => static code specific to a product reference
UDI-PI = i“production” identification => dynamic code specific to 1 lot or serial no. (lot no., expiry date, etc.)
The Basic UDI-DI is a regulatory identifier not meant to be affixed to the device. It encompasses devices that have:
- the same purpose,
- the same risk class, and
- the same essential design and manufacturing characteristics.
The UDI and Basic UDI-DI shall apply to all MDs, except for custom-made MDs and those undergoing clinical investigation.
Timetable for implementation:
Creation of Basic UDI-DIs and UDI-DIs:
- Basic UDI-DIs: for MDR/IVDR CE certification.These codes shall be present in technical documentation and on MDR/IVDR CE certificates. It is not necessary to create a Basic UDI-DI for legacy devices* certified under Directive 93/42/EEC. Indeed, a “Eudamed-DI” code may be created via the Eudamed database (https://ec.europa.eu/health/sites/default/files/md_eudamed/docs/legacy_dvc_management_en.pdf). In all cases, the same device may not keep the same Basic UDI-DI when it is MDR-certified.
- UDI-DIs: at the latest for MDR/IVDR CE certification and the insertion of codes in Eudamed.These codes may be in the technical documentation and MDR/IVDR certificates and will be one of the attributes to be entered in Eudamed. The UDI does not have to be affixed to the device for legacy devices*.
Insertion of Basic UDI-DI codes (and UDI-DI codes) in Eudamed:
- Voluntary: September 2021.
- Mandatory: within 24 months after the Eudamed database becomes fully functional, or if a case of vigilance is notified via this database, when Eudamed is not fully functional.
Affixing of UDIs to products (UDI-DI and UDI-PI):
- MDR-certified MDs (Article 123(3)(f), Article 27(4)):
- Implantable & Class III MDs: 26 May 2021
- Class IIa & IIb MDs: 26 May 2023
- Class I MDs: 26 May 2025
- Direct marking on reusable MDs (MDR Article 123(3)(g), Article 27(4)):
- Implantable & Class III MDs: 26 May 2023
- Class IIa & IIb MDs: 26 May 2025
- Class I MDs: 26 May 2027
- IVDR-certified IVDMDs (Article 113(3)(e), Article 24(4)):
- Class D IVDMDs: 26 May 2023
- Class C and B IVDMDs: 26 May 2025
- Class A IVDMDs: 26 May 2027
Systems and procedure packs are also concerned:
The assembler (person referred to in Article 22 of the MDR) must define and assign a Basic UDI and a UDI for their system(s) and procedure pack(s).
After registering as an economic operator, the assembler will have to register their systems and procedure packs in Eudamed.
The Efor Group assists you with the following aspects of compliance:
– Compliance strategy
– Operational support for quality, regulatory and engineering aspects
– Validation of computerised systems
[1] Legacy Devices : DM conformes à la Directive 93/42/CEE ou 90/385/CEE, pouvant continuer à être mis sur le marché après le 26 mai 2021 en vertu de l’article 120(3) du règlement (UE) 2017/745 (RDM), ou les DMDIV conformes à la Directive 98/79/CE pouvant continuer à être mis sur le marché après le 26 mai 2022 en vertu de l’article 110(3) du règlement (UE) 2017/746 (RDMDIV).
Efor group
Our CSR commitments
Aware of our social and environmental responsibility, we act every day to make a positive impact on society.
Nos actualités
Suivez toutes nos infos santé


