DMDIV: Gradual implementation of regulation (EU) 2017/746
7/07/2022

Since 26 May 2002, the placing on the European market of all IVDMDs has been subject to compliance with Regulation (EU) 2017/746 “on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU”.
Regulation (EU) 2017/746 introduces major changes in the area of in vitro diagnostic medical devices (IVDMDs). The Regulation aims to ensure the smooth functioning of the internal market and a high level of protection of public health for patients and users.
One of the main changes relates to the intervention of independent compliance assessment organisations (notified bodies). Under Directive 98/79/EC, only a relatively small number of high-risk devices (around 8% of all the IVDMDs available on the market) were subject to oversight by notified bodies. According to the Regulation, around 80% of them will be supervised by notified bodies, the large majority for the first time.
Transitional provisions for IVDMDs that were CE marked before 26 May 2022 are presented in Article 110 of Regulation (EU) 2017/746. Following the publication of Regulation (EU) 2022/112, the transitional periods have been extended depending on the risk class of the device concerned. They are now shorter for devices belonging to a higher risk class and longer for those belonging to a lower risk class.
The lengths of the transitional periods are set out below:
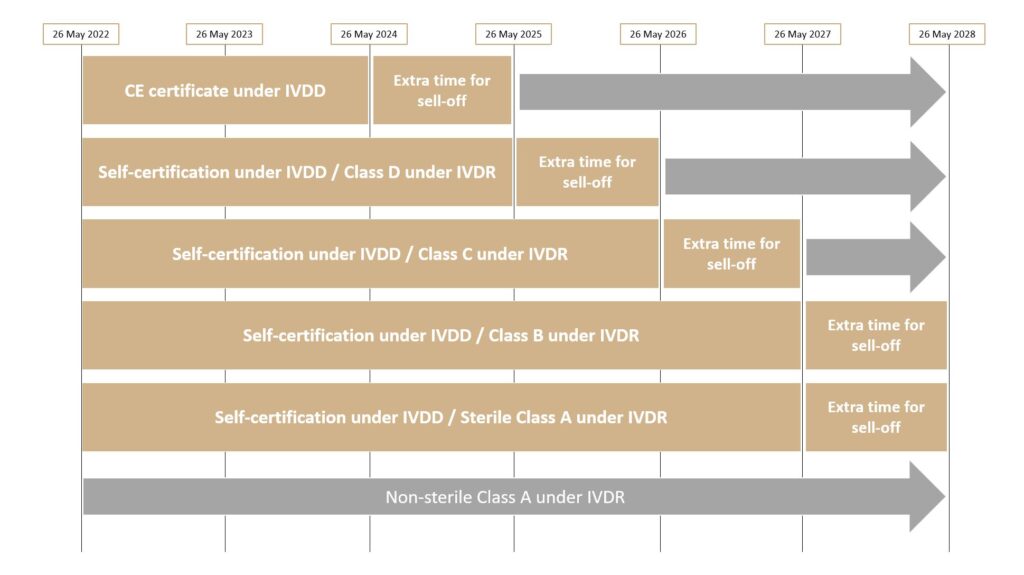
For Class D and C devices, the transitional period runs until May 2025 and May 2026 respectively while for Class B and A devices, it ends in May 2027.
For devices that cannot benefit from a transitional period, such as Class A devices and new IVDMDs, they must have been compliant with Regulation (EU) 2017/746 since 26 May 2022.
Our intervention
Since the introduction of Regulation (EU) 2017/746, new deliverables have been required that are part of the technical documentation to be submitted to the notified body (scientific validity, performance evaluation plan and report, etc.).
The EFOR Group provides its customers/IVDMD manufacturers, importers & distributors with its expertise and a methodical approach. It has a team of specialised experts who will support them through all the steps of the IVDR transition.
Efor group
Our CSR commitments
Aware of our social and environmental responsibility, we act every day to make a positive impact on society.
Nos actualités
Suivez toutes nos infos santé


