DM/DMDIV: Analysis and trend reports during post-marketing surveillance
30/11/2022

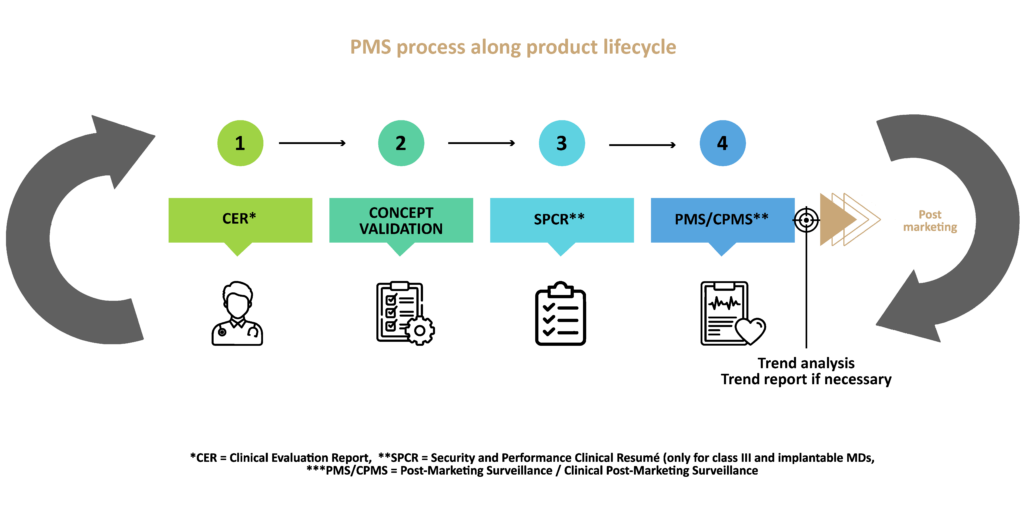
The Medical Devices Regulations (MDR) 2017/745 and the In Vitro Diagnostic Medical Devices (IVDR) Regulation 2017/746 require the implementation of a proactive post-market surveillance system (PMS) by the manufacturer.
Among the new obligations of this process, a new requirement brought by the RDM and IVDR is the trend report.
The requirements of regulations 2017/745 and 2017/746
The SAC plan, referred to in Article 84 of the MDR, must include a methodology to identify any statistically significant increase in the frequency or severity of incidents that are not defined as serious incidents, or expected adverse side effects with significant impact on the benefit/risk ratio.
The requirements of the RDIV are very similar. The SAC plan referred to in article 83 of the IVDR must also include a methodology to identify any statistically significant increase in the frequency or severity of incidents that are not defined as serious incidents, and could have a significant impact on the benefit/risk ratio. In addition to that, any significant increase in expected results that do no meet the claimed performance of the device.
If this increase is significant, the manufacturer must set up a trend report as required in article 88. The report must be sent to the competent authorities via Eudamed (or directly to the authorities as long as the Eudamed database is not not fully functional – Cf MDCG 2021-1 Rev 1 and MDCG 2022-12).
Analyze trends
In order to set up a trend analysis, certain amount of data is necessary to demonstrate a statistically significant increase. The statistical method and the minimum amount of data required should be clearly defined in your SAC plan. The type of data collected and its analysis will depend at least on the following points:
- the type of product,
- regulatory status,
- input data,
- The level of risk.
All methods of analysis must establish a definition of thresholds or limits not to be exceeded. The thresholds are defined according to one or more parameters related to performance or security and defined by the manufacturer. We will therefore process raw data to then have averages, frequencies, standard deviations, etc.
The representation of this tendency can be expressed in different ways. It is often easier to use a graph to follow the evolution of the results.
Following the trend analysis, if a statistically significant trend emerges, the manufacturer must provide an action plan for the corrective measures intended and the results of this analysis in a trend report.
Finally, the trend analysis makes it possible to update the risk analysis of the product and the evaluation of the risk-benefit ratio, and thus decide on the performance and safety of the product.
Our action:

The implementation of such process is a challenge for manufacturers. Efor-CVO assists you by offering its service for the full implementation of your SAC quality system (procedures, plans and reports) in order to ensure its compliance.
Our team of experienced statisticians will be a major asset in validating your trend methods and adapting them to your products.
Efor group
Our CSR commitments
Aware of our social and environmental responsibility, we act every day to make a positive impact on society.
Nos actualités
Suivez toutes nos infos santé


